Electrocatalytic determination of maltol in food products by cyclic voltammetry with a poly(l-phenylalanine) modified electrode
Abstract
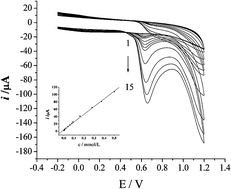
Herein, a poly(L-phenylalanine) modified glassy carbon electrode (PLPA/GCE) was fabricated by electrochemical polymerization and a convenient method for the determination of maltol in food products was developed using the fabricated PLPA/GCE. The electrochemical behavior of maltol at the modified electrode was studied by cyclic voltammetry. Experimental results show that the modified electrode exhibits excellent electrocatalytic activity towards the electrochemical oxidation of maltol and the oxidation is a one-proton–one-electron process. In pH 8.0 phosphate buffer solution, the oxidation peak current obtained by cyclic voltammetry is linearly proportional to the concentration of maltol in the range of 8.0 × 10−7 to 5.00 × 10−4 mol L−1 with a limit of detection of 9.5 × 10−8 mol L−1. The stability and reproducibility of the fabricated electrode was evaluated and the performance of the proposed method was validated in terms of linearity (r = 0.9989), recovery (95.2–101.4%), reproducibility (RSD < 3.7%, n = 6) and robustness. The applicability of the developed method has been successfully demonstrated by the determination of maltol in a variety of food products such as cake, beer and red wine.

 Please wait while we load your content...
Please wait while we load your content...