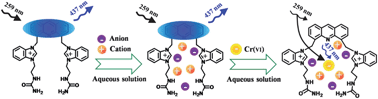
In this article, a facile and versatile strategy for highly selective detection of Cr(VI) in 100% aqueous solution was developed based on fluorescence inner filter effect (IFE) using a hydrophilic ionic chemosensor. In the presence of Cr(VI), remarkable fluorescence quenching of the chemosensor could be observed because of the strong absorption of Cr(VI) to both the excitation and emission light of the fluorophore (in this work, acridine was chosen as the model fluorophore). By utilizing the modified Benesi–Hildebrand equation, not only was the efficiency of IFE quantitatively calculated, but also the calibration curve used for Cr(VI) determination was vastly broadened. A linear range of 5.0 × 10−6 to 1.4 × 10−3 M for Cr(VI), along with a detection limit of 8.9 × 10−7 M and a RSD of 3.6% at 2.5 × 10−5 M in 100% aqueous solution were obtained. In addition, the detection limit could be as low as 9.2 × 10−8 M in MeCN aqueous solution. Meanwhile, the present method possesses an excellent selectivity for Cr(VI) detection. It was also successfully applied in Cr(VI)-spiked tap water samples and wastewater samples.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...