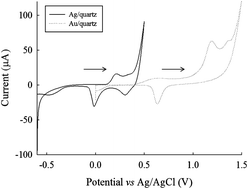
We studied the electrochemical behavior of 2-mercaptothiazoline (1,3-thiazolidine-2-thione) on silver (Ag)/quartz and gold (Au)/quartz electrodes electrochemically deposited it on silver metal film on Ag/quartz substrates, and investigated it using electrochemical quartz crystal microbalance (EQCM) analysis with AT-cut quartz crystals (9 MHz). We found that the electrocatalytic property of Ag/quartz was better than that of gold/quartz. In addition, 2-mercaptothiazoline was detected using high-performance liquid chromatography with an electrochemical (Ag) detector (HPLC-ECD). For direct current mode, with the current at a constant potential, and measurements with suitable experimental parameters, we found a linear concentration range from 0.01 to 0.80 μg mL−1. The lower limit of detection using our method was approximately 0.1 ng. Findings using HPLC-ECD and HPLC with an ultraviolet detector were comparable.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...