A novel detection of nitrite, iodate and bromate based on a luminescent polyoxometalate†
Abstract
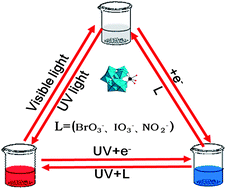
In this paper, we describe a novel detection of nitrite, iodate and bromate in solutions through combining the electrochemistry and the redox reaction accompanying the color change and luminescence switching based on Eu3+-containing tungstogermanate [(CH3)4N]2.5H7.5[Eu(GeW11O39)(H2O)2]2·4.5H2O (Eu-POM) for the first time. Eu-POM was electroreduced to form the reduced Eu-POM accompanied by a color change from colorless to blue and luminescence quenching, while the discoloration and luminescence recovery were observed after the addition of nitrite, iodate and bromate because of the spontaneous redox reactions of nitrite, iodate and bromate with the reduced Eu-POM. Based on this observation, we investigated the changes of absorbances and luminescence intensities of the reduced Eu-POM with the concentrations of nitrite, iodate and bromate and obtained good linear ranges and lower detection limits for the three substances based on S/N = 3. This method successfully combines the electrochromic and luminescent properties of polyoxometalate to develop a novel detection of nitrite, iodate and bromate with good reversibility and high sensitivity as well as a wide linear range. Therefore, such as system shows great potential for the detection of nitrite, iodate and bromate.

 Please wait while we load your content...
Please wait while we load your content...