Determination of uric acid in biological samples on the pretreated pencil graphite electrode
Abstract
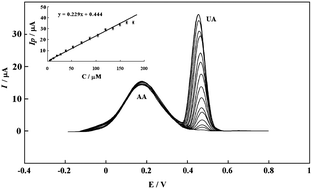
The electrochemical behavior of uric acid (UA) has been investigated on the pretreated pencil graphite electrode (PPGE). PPGE was prepared by potentiodynamic pretreatment of pencil lead in phosphate buffer solution (pH 7). On non-pretreated pencil graphite electrode (NPGE), ascorbic acid (AA) and uric acid (UA) both exhibit totally irreversible anodic peaks which overlap each other. While on PPGE, anodic peak of AA shifts to negative potentials with a decrease in peak current; but that of UA exhibits only an increase in peak current. This behavior on PPGE results in significant resolution of UA and AA peaks, which provides the voltammetric determination of UA in the presence of AA (ΔEp ≈ 320 mV). The electrochemical oxidation of UA on PPGE being a totally irreversible process is controlled by diffusion. The kinetic parameters such as charge transfer coefficient, α, and catalytic rate constant, kh, were determined for UA at PPGE. At optimum conditions of solution pH and pulse amplitude, differential pulse voltammetry (DPV) was used for determination of UA, which exhibits a linear calibration graph of Ipversus UA concentration in the range of 1–160 μM with a correlation coefficient of 0.997. The calculated detection limit for S/N = 3 was 1.9 × 10−7 M. Finally, PPGE was used for determination of UA in biological samples such as human serum and urine, using the standard addition procedure. The obtained results were compared with those of standard spectrophotometric method, which reveals that no significant difference exists at 95% confidence level between the results of these two procedures.

 Please wait while we load your content...
Please wait while we load your content...