Progress curve analysis of enzyme reactions by flow microcalorimetry: Use of a pseudo-first order approximation
Abstract
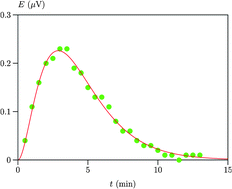
We describe a method for determining the pseudo-first order rate constant of a chemical reaction by flow microcalorimetry operating in the flow-through mode. The impulse response of the instrument was described by a Gamma distribution and the equation of the

 Please wait while we load your content...
Please wait while we load your content...