A specific electrochemiluminescence sensor for selective and ultra-sensitive mercury(ii) detection based on dithiothreitol functionalized copper nanocluster/carbon nitride nanocomposites†
Abstract
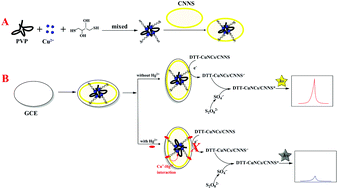
Electrochemiluminescence (ECL) sensors are useful for the detection of heavy metal pollutants, in particular mercury(II) ions, in water samples. We demonstrate the superior sensing performance of Hg2+ using a nanocomposite material based on carbon nitride nanosheets (CNNSs) and copper nanoclusters functionalized by dithiothreitol, which not only stabilizes the clusters, but also improves the sensitivity of Hg2+ detection. The ECL mechanism is related to the reaction of the nanocomposite with K2S2O8 in the electrochemical system, while the presence of Hg2+ leads to quenching of its excited state, and the suppression of the formation of anion-radicals. The Hg(II) sensor presented here is cheap and fast, and shows high selectivity for the detection of Hg2+ on the background of other mono-, di-, and trivalent ions, with a linear range of 0.5–10 nM and the detection limit as low as 0.01 nM.


 Please wait while we load your content...
Please wait while we load your content...