Native mass spectrometry beyond ammonium acetate: effects of nonvolatile salts on protein stability and structure†
Abstract
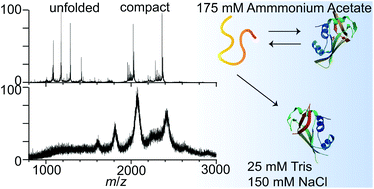
Native mass spectrometry is widely used to probe the structures, stabilities, and stoichiometries of proteins and biomolecular complexes in aqueous solutions, typically containing volatile ammonium acetate or ammonium bicarbonate buffer. In this study, nanoelectrospray emitters with submicron tips are used to produce significantly desalted ions of RNase A and a reduced, alkylated form of this protein, RA-RNase A, from solutions containing 175 mM ammonium acetate, as well as sodium chloride and Tris containing solutions with the same nominal ionic strength and pH. The charge-state distributions formed by nanoelectrospray ionization and tyrosine fluorescence emission data as a function of temperature from these solutions indicate that the folded form of RA-RNase A in solution is stabilized when ammonium acetate is replaced by increasing quantities of NaCl and Tris. Ion mobility data for the 7+ charge state of RA-RNase A indicates that the protein conformation in ammonium acetate changes with increasing concentration of NaCl which stablizes more compact structures. These results are consistent with observations reported 130 years ago by Hofmeister who found that ion identity can affect the stabilities and the structures of proteins in solution. This study indicates the importance of buffer choice when interpreting native mass spectrometry data.


 Please wait while we load your content...
Please wait while we load your content...
