Reaction-based fluorometric analysis of N-bromosuccinimide by oxidative deprotection of dithiane†
Abstract
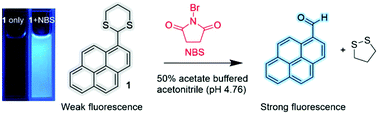
In this study, a fluorescent probe is developed for the first time for N-bromosuccinimide (NBS), a synthetically and analytically important compound. Pyrene–dithiane-based probe 1 showed prominently selective and sensitive signaling behavior toward NBS owing to the oxidative cleavage of the dithiane protecting group of 1-pyrenecarboxaldehyde. The NBS-selective signaling of the probe was possible under competitive conditions in the presence of common metal ions and anions as a background. The detection limit of the probe for NBS was found to be 5.6 × 10−8 M (10.0 ppb). The signaling product was sufficiently stable under the oxidative stress of NBS in contrast to another tested compound, 6-methoxy-2-naphthaldehyde-based dithiane derivative 2, which showed a gradually decaying response because of the reaction of the signaling product with the residual NBS. In the practical application of the probe, a smartphone was used as a stand-alone device, and the fluorometric assays of the commercial NBS reagents could be conducted rapidly and in a convenient manner.


 Please wait while we load your content...
Please wait while we load your content...