Different binding sites of serum albumins in the protein corona of gold nanoparticles†
Abstract
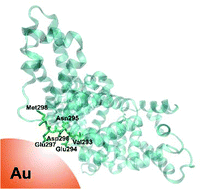
The interaction of bovine serum albumin (BSA) and human serum albumin (HSA), sharing a sequence similarity of 77.5%, with gold nanoparticles of a size of ∼30 nm was investigated by surface-enhanced Raman scattering (SERS). The spectra provide information on those residues of the proteins in proximity of the nanoparticles. The SERS signals indicate an electrostatic interaction of both proteins with the citrate ligands at the nanoparticle surface via lysine residues. HSA, different from BSA also binds directly to the gold surface by particularly flexible protein segments that were identified by comparison of the vibrational bands with the known amino acid sequence of the molecule. The data suggest that both the direct binding as well as interaction with the citrate ligands determine the interaction, yet to varying extent in the two very similar serum proteins. This has implications for their use in bio-functionalization, and for the application of gold nanostructures in bioanalytics and medicine.
- This article is part of the themed collections: Clinical spectroscopy and SPEC 2018: International Society of Clinical Spectroscopy


 Please wait while we load your content...
Please wait while we load your content...