Vibrational spectroscopic monitoring and biochemical analysis of pericellular matrix formation and maturation in a 3-dimensional chondrocyte culture model
Abstract
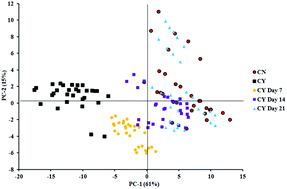
Isolated and monolayer expanded chondrocytes are not the ideal cell form to produce a cartilage matrix. In articular cartilage, each chondrocyte is surrounded by a 2–4 μm thick collagen VI-rich pericellular matrix (PCM) forming a chondron. Freshly extracted chondrons form a more cartilage-like extracellular matrix (ECM) than chondrocytes and their surrounding PCM is thought to maintain the chondrocyte phenotype. To regenerate articular cartilage, preserving and/or regenerating a functional PCM is essential. In this study, a highly biomimicking hyaluronic acid (HA) hydrogel was used as a 3-dimensional system to culture freshly isolated bovine chondrons (with an intact PCM) and chondrocytes (without a PCM) for up to 21 days. We assessed the HA hydrogel's capacity to maintain and potentially re-generate PCM formation by both biochemical and immunological analyses of the key components of the PCM. For the first time, synchrotron based Fourier transform infrared (SR-FTIR) microspectroscopy was utilised to reveal the dynamic process of PCM re-generation. At day 1, highly specific collagen VI staining was visible within chondron containing HA hydrogels. In contrast, collagen VI was absent at day 1 but punctate, focal staining increased during the culture period of chondrocyte containing HA hydrogels. Chondron containing HA hydrogels produced more collagen II and GAGs than the chondrocyte containing HA hydrogels. Principal component analysis (PCA) of spectra in fingerprint regions of the chondrocyte-containing constructs at day 7, 14 and 21 culturing showed clear spectral differences. The clusters of day 14 and day 21 samples were closer to the chondron samples, while the day 7 samples were closer to chondrocytes. PCA scores in the lipid region revealed no major differences between chondrocyte and chondron samples, but showed that the cultured chondrocyte samples at day 7, day 14 and day 21 clustered together. These data would indicate that SR-FTIR microspectroscopy can help to better understand the PCM formation and maturation in tissue engineered models, which involves subtle changes in collagen and aggrecan.
- This article is part of the themed collections: Clinical spectroscopy and SPEC 2018: International Society of Clinical Spectroscopy


 Please wait while we load your content...
Please wait while we load your content...