Monitoring individual cell-signaling activity using combined metal-clad waveguide and surface-enhanced fluorescence imaging
Abstract
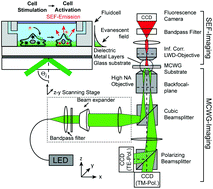
Evanescent field based biosensing systems such as surface plasmon resonance (SPR), diffraction gratings, or metal-clad waveguides (MCWGs) are powerful tools for label-free real-time monitoring of signaling activity of living cells exposed to hormones, pharmacological agents, and toxins. In particular, MCWG-based imaging is well suited for studying relatively thick objects such as cells due to its greater depth of penetration into the sensing medium, compared to SPR. Label-free methods, however, provide only indirect measurements in that the measured signal arises from local changes in material properties rather than from specific biomolecular targets. In the case of cells, the situation is especially complex as the measured label-free signal may result from a combination of very diverse sources: morphological changes, intra-cellular reorganization, cascaded molecular events, protein expression etc. Consequently, deconvolving the contributions of specific sources to a particular cell response profile can be challenging. In the following, we present a cell imaging platform that combines two distinct sensing modalities, namely label-free MCWG imaging and label-based surface enhanced fluorescence (SEF), designed to facilitate the identification of the underlying molecular and structural contributions to the label-free MCWG images. We demonstrate the bimodal capabilities of this imaging platform in experiments designed to visualize actin cytoskeleton organization in vascular smooth muscle cells. We then monitored the real-time response of HEK293 cells expressing the Angiotensin 1 receptor (AT1R), when stimulated by the receptor agonist Angiotensin II (AngII). The analysis of the simultaneous label-free signal obtained by MCWG and the intracellular calcium signal resulting form AT1R activation, measured by SEF, allows relating label-free signal features to specific markers of receptor activation. Our results show that the intracellular calcium levels normally observed following AT1R activation are not required for the initial burst of cellular activity observed in the MCWG signal but rather indicates signaling activity involving the intracellular kinase ROCK.


 Please wait while we load your content...
Please wait while we load your content...