Continuous amperometric hydrogen gas sensing in ionic liquids†
Abstract
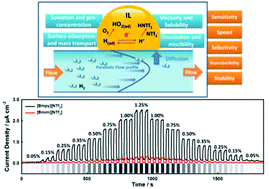
We report an innovative amperometric hydrogen sensor that addresses current primary issues (i.e. signal drift, low selectivity and speed) in continuous and real-time gas sensing. Utilizing the unique properties and redox reactions of hydrogen in the ionic liquids (ILs), 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide [Bmpy][NTf2] and 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide [Bmim][NTf2], we demonstrate the real-time and continuous sensing of hydrogen with high sensitivity, selectivity and repeatability in both anaerobic and aerobic conditions using simple constant potential amperometry. The varying adsorption of hydrogen at the IL-electrode interface in different ILs is shown to allow tuning of the sensitivity of the sensor. Taking advantage of oxygen in ambient conditions, we demonstrate that the unique chemical reaction of the analyte with the oxygen enables selective quantification of hydrogen in an ambient environment. A sensor calibration based on a kinetics analysis (i.e. the change of the rate of current signal (ΔI/Δt1/2)) rather than an equilibrium analysis was demonstrated to allow fast and quantitative analysis of hydrogen concentration. The ionic liquid hydrogen sensor exhibits high sensitivity, selectivity, speed, accuracy, repeatability and stability. Together with the miniaturization and affordability of amperometric sensor readout electronics, the IL-based electrochemical gas sensor is expected to enable area-wide sensing instead of point measurements for environmental, health and occupational safety applications.


 Please wait while we load your content...
Please wait while we load your content...