Ionophore-based optical nanosensors incorporating hydrophobic carbon dots and a pH-sensitive quencher dye for sodium detection†
Abstract
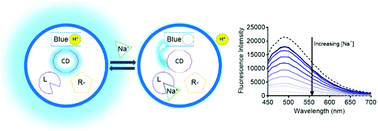
Nanosensors present a biological monitoring method that is biocompatible, reversible, and nano-scale, and they offer many advantages over traditional organic indicators. Typical ionophore-based nanosensors incorporate nile-blue derivative pH indicators but suffer from photobleaching while quantum dot alternatives pose a potential toxicity risk. In order to address this challenge, sodium selective nanosensors containing carbon dots and a pH-sensitive quencher molecule were developed based on an ion-exchange theory and a decoupled recognition element from the pH indicator. Carbon dots were synthesized and integrated into nanosensors containing a pH-indicator, an analyte-binding ligand (ionophore), and a charge-balancing additive. These nanosensors are ion-selective against potassium (selectivity coefficient of 0.4) and lithium (selectivity coefficient of 0.9). Reversible nanosensor response to sodium is also demonstrated. The carbon dot nanosensors are resistant to changes in optical properties for at least 12 h and display stable selectivity to physiologically-relevant sodium (alpha = 0.5 of 200 mM NaCl) for a minimum of 6 days.


 Please wait while we load your content...
Please wait while we load your content...