Limited proteolysis in porous membrane reactors containing immobilized trypsin†
Abstract
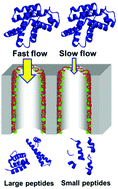
Proteolysis is often a critical step in protein characterization via mass spectrometry. Compared to complete digestion, limited proteolysis gives larger peptides, and the dominant cleavage sites may identify highly accessible, flexible protein regions. This paper explores controlled proteolysis in porous nylon membranes containing immobilized trypsin. Passage of protein solutions through ∼100 μm thick membranes provides reaction residence times as short as milliseconds to limit digestion. Additionally, variation of the membrane pore size and the protease-immobilization method (electrostatic adsorption or covalent anchoring to adsorbed polymer in membrane pores) affords control over the proteolysis rate. When digesting the highly labile protein β-casein, large membrane pores (5.0 μm) and covalent enzyme anchoring to adsorbed polymer lead to particularly long tryptic peptides. With the more trypsin-resistant proteins cytochrome c and apomyoglobin, in-membrane proteolysis with short residence times, 1.2 μm membrane pores, and trypsin electrostatically immobilized to an adsorbed polyanion cleaves the proteins after lysine residues in flexible regions. For both cytochrome c and apomyoglobin, cleavages in an interhelix region yield two particularly large peptides that cover the entire protein sequence.


 Please wait while we load your content...
Please wait while we load your content...