Fourier-transform infrared spectroscopy for characterization of protein chain reductions in enzymatic reactions†
Abstract
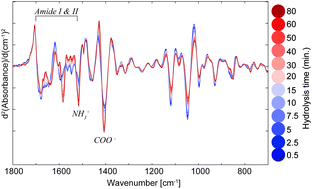
The potential of dry-film Fourier-transform infrared (FTIR) measurements as a monitoring tool for enzymatic hydrolysis of protein-based substrates is explored in this study. As a proof-of-concept, the enzymatic digestion of bovine serum albumin using Alcalase was monitored. To evaluate the analytical approach on complex substrates with industrial relevance, salmon- and chicken-based substrates were digested for 80 minutes using Alcalase and a total of 12 FTIR spectra were acquired during the course of the hydrolysis. The observed changes in the IR spectral features as a function of hydrolysis time were found to be in agreement with the breakdown of the amide backbone and formation of amino and carboxylate terminals. Some of the most consistent markers for hydrolysis time were the bands at 1516 cm−1 (–NH3+) and ∼1400 cm−1 (–COO−). Moreover, principal component analysis (PCA) of the FTIR spectra was used to demonstrate the systematic relationship of the hydrolysis time with key variables (wavelengths) in the protein backbone region (800–1800 cm−1). Scores in the first principal component versus the hydrolysis time have been shown to provide an overview of the process dynamics related to protein structural changes. The herein presented results suggest that dry-film FTIR measurements have potential as a rapid tool for monitoring industrial protein hydrolysis processes.


 Please wait while we load your content...
Please wait while we load your content...