Nanospray HX-MS configuration for structural interrogation of large protein systems†
Abstract
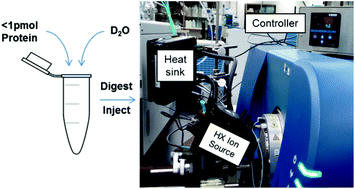
Hydrogen-deuterium exchange mass spectrometry (HX-MS) has made important contributions to the study of protein structure and function. Unfortunately, it is not known for low limits of detection, when compared with other forms of peptide-based or bottom-up protein MS methods. Systems perform poorly on sub-pmol quantities of protein states with greater than 300 kDa of unique sequences. The HX-MS analysis of complex protein states would be possible if proteomics-grade configurations could be used reliably, but temperature and temporal constraints have proven to be significant design challenges. Here, we describe an integrated HX-MS ion source operating on a vented-column geometry, which brings regulated column cooling right to the spray tip. The design offers chromatographic peak widths of 2–6 s (FWHM). It provides stable operation at 500 nL min−1, while retaining deuteration levels comparable to conventional geometries. We demonstrate at least a 50-fold improvement in protein consumption levels, and illustrate robustness by measuring peptide-averaged protection factors for 90% of DNA-PKcs, a 469 kDa protein, from 0.5 pmol injections.


 Please wait while we load your content...
Please wait while we load your content...