Improving sensitivity and linear dynamic range of intact protein analysis using a robust and easy to use microfluidic device
Abstract
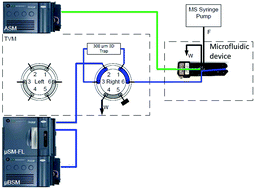
We demonstrate an integrated microfluidic LC device coupled to a QTOF capable of improving sensitivity and linearity for intact protein analysis while also tuning the charge state distributions (CSD) of whole antibodies. The mechanism for sensitivity improvement using microflow ESI is demonstrated by shifting of the CSD to higher charge state, and narrowing of the overall CSD. Both of these aspects serve to improve ion current of the most abundant charge state of antibodies and lead to improvement in sensitivity over high flow ESI by a factor of 15×. Current limits of detection are 0.1 ng (on-column) (n = 100, %RSD = 17.5) using IgG glycosylated antibody, as compared to 5 ng (on-column) (n = 10, %RSD = 15) for high flow LC-ESI-MS. In addition to improvements in sensitivity we also observe improvements in linear dynamic range for microflow ESI that results from a combination of lower limits of detection and narrower CSD. An improvement of linear dynamic range of 1.5 orders of magnitude was observed over conventional high flow LC-MS. In cases where the complexity of the antibody limited both sensitivity and spectral charge state resolution, we employed supercharging and decharging mechanisms to further improve sensitivity and charge state spacing resolution. We demonstrate an 89% increase in sensitivity using glycerol that was added post column, with retention of the glycoform resolution. Since large proteins reside in a relatively low noise region of the mass spectra it is possible to realize effects of supercharging for intact proteins, specifically antibodies of 150 kDa, that are less pronounced for peptide supercharging. We also demonstrate a 51% increase in charge state resolution as imidazole was used to generate lower charge states for high-mass ions. The increase in charge state resolution enables more complex antibodies, or antibody mixtures that coelute in the LC, to be deconvoluted more efficiently. In summary, we demonstrate an analytical technique that yields improved sensitivity and quantitative linear dynamic range for intact protein analysis over conventional LC-MS, and yields ease of use for more complex experimentation such as supercharging and decharging experiments.


 Please wait while we load your content...
Please wait while we load your content...