Abstract
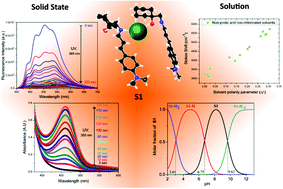
Non-classical protomerism of Schiff bases offers several advantages; for example, specific interactions in the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– linkage can be controlled and differentiated because the interactions are not governed by keto–enol tautomerism. Herein, the pH sensing properties of a new protomeric Schiff base probe (S1) are reported. In particular, among several acids, the probe displays significant optical responses upon interaction with hydrochloric acid (HCl). X-ray structural analysis confirmed the existence of an intermolecular interaction with HCl through a –C
N– linkage can be controlled and differentiated because the interactions are not governed by keto–enol tautomerism. Herein, the pH sensing properties of a new protomeric Schiff base probe (S1) are reported. In particular, among several acids, the probe displays significant optical responses upon interaction with hydrochloric acid (HCl). X-ray structural analysis confirmed the existence of an intermolecular interaction with HCl through a –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N⋯H–Cl⋯O– linkage. Moreover, an optical response via a second channel is manifested as photochromic fluorescence behavior. The properties of S1 were investigated by UV-vis and fluorescence spectroscopy in a solution and the solid state. Its strong acidofluorochromic behavior was analyzed and its pKa and
N⋯H–Cl⋯O– linkage. Moreover, an optical response via a second channel is manifested as photochromic fluorescence behavior. The properties of S1 were investigated by UV-vis and fluorescence spectroscopy in a solution and the solid state. Its strong acidofluorochromic behavior was analyzed and its pKa and 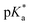 values were determined, which revealed a photobasic character. Positive solvatochromism that resulted from specific interactions taking place in S1 was studied using four different solvent scales, namely, Lippert–Mataga, Kamlet–Taft, Catalán and the recently proposed scale of Laurence et al., which yielded consistent results. Finally, theoretical calculations were conducted to analyze the mechanism of the probe in terms of natural transition orbitals (NTOs) and the spatial extent of charge transfer excitations.
values were determined, which revealed a photobasic character. Positive solvatochromism that resulted from specific interactions taking place in S1 was studied using four different solvent scales, namely, Lippert–Mataga, Kamlet–Taft, Catalán and the recently proposed scale of Laurence et al., which yielded consistent results. Finally, theoretical calculations were conducted to analyze the mechanism of the probe in terms of natural transition orbitals (NTOs) and the spatial extent of charge transfer excitations.

 Please wait while we load your content...
Please wait while we load your content...