Polythiophene derivative on quartz resonators for miRNA capture and assay†
Abstract
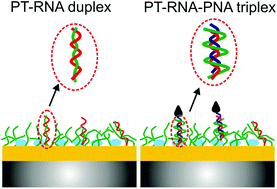
A novel approach for miRNA assay using a cationic polythiophene derivative, poly[3-(3′-N,N,N-triethylamino-1′-propyloxy)-4-methyl-2,5-thiophene hydrobromide] (PT), immobilized on a quartz resonator is proposed. The cationic PT enables capturing of all RNA sequences in the sample matrix via electrostatic interactions, resulting in the formation of PT–RNA duplex structures on quartz resonators. Biotinylated peptide nucleic acid (b-PNA) sequences are subsequently utilized for the RNA assay, upon monitoring the PT–RNA–b-PNA triplex formation. Signal amplification is achieved by anchoring avidin coated nanoparticles to b-PNA in order to yield responses at clinically relevant concentration regimes. Unlike conventional nucleic acid assay methodologies that usually quantify a specific sequence of RNA, the proposed approach enables the assay of any RNA sequence in the sample matrix upon hybridization with a PNA sequence complementary to the RNA of interest. As an illustration, successful detection of mir21, (a miRNA sequence associated with lung cancer) is demonstrated with a limit of detection of 400 pM. Furthermore, precise quantification of mir21 in plasma samples is demonstrated without requiring PCR and sophisticated instrumentation.


 Please wait while we load your content...
Please wait while we load your content...