Affinity imaging mass spectrometry (AIMS): high-throughput screening for specific small molecule interactions with frozen tissue sections†
Abstract
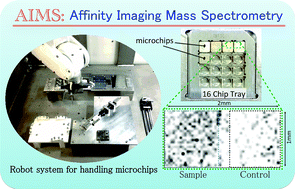
A novel screening system, using affinity imaging mass spectrometry (AIMS), has been developed to identify protein aggregates or organ structures in unfixed human tissue. Frozen tissue sections are positioned on small (millimetre-scale) stainless steel chips and incubated with an extensive library of small molecules. Candidate molecules showing specific affinity for the tissue section are identified by imaging mass spectrometry (IMS). As an example application, we screened over a thousand compounds against Alzheimer's disease (AD) brain tissue and identified several compounds with high affinity for AD brain sections containing tau deposits compared to age-matched controls. It should also be possible to use AIMS to isolate chemical compounds with affinity for tissue structures or components that have been extensively modified by events such as oxidation, phosphorylation, acetylation, aggregation, racemization or truncation, for example, due to aging. It may also be applicable to biomarker screening programs.
- This article is part of the themed collection: Alzheimer's Research Month 2016

 Please wait while we load your content...
Please wait while we load your content...