Early stages of insulin fibrillogenesis examined with ion mobility mass spectrometry and molecular modelling†
Abstract
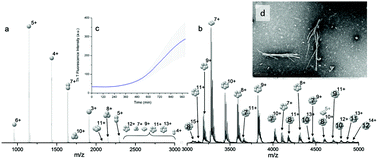
A prevalent type of protein misfolding causes the formation of β-sheet-rich structures known as amyloid fibrils. Research into the mechanisms of fibril formation has implications for both disease prevention and nanoscale templating technologies. This investigation into the aggregation of insulin utilises ion mobility mass spectrometry coupled with molecular modelling to identify and characterise oligomers formed during the ‘lag’ phase that precedes fibril growth. High resolution mass spectrometry and collision induced dissociation is used to unequivocally assign species as m/z coincident multimers or confomers, providing a robust analytical approach that supports the use of molecular dynamics to atomistically resolve the observed oligomers. We show that insulin oligomerises to form species In where 2 ≤ n ≤ 12 and within this set of oligomers we delineate over 60 distinct conformations, the most dominant of which are compact species. Modelling trained with experimental data suggests that the dominant compact dimers are enriched in β-sheet secondary structure and dominated by hydrophobic interactions, and provides a linear relationship between Rg and collision cross section. This approach provides detailed insight to the early stages of assembly of this much studied amyloidogenic protein, and can be used to inform models of nucleation and growth.
- This article is part of the themed collection: Ion Mobility Mass Spectrometry

 Please wait while we load your content...
Please wait while we load your content...