19F NMR-, ESR-, and vis-NIR-spectroelectrochemical study of the unconventional reduction behaviour of a perfluoroalkylated fullerene: dimerization of the C70(CF3)10− radical anion†
Abstract
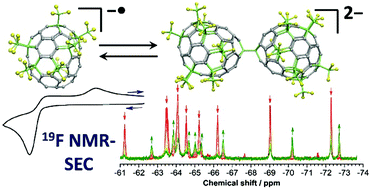
The most abundant isomer of C70(CF3)10 (70-10-1) is a rare example of a perfluoroalkylated fullerene exhibiting electrochemically irreversible reduction. We show that electrochemical reversibility at the first reduction step is achieved at scan rates higher than 500 V s−1. Applying ESR-, vis-NIR-, and 19F NMR-spectroelectrochemistry, as well as mass spectrometry and DFT calculations, we show that the (70-10-1)− radical monoanion is in equilibrium with a singly-bonded diamagnetic dimeric dianion. This study is the first example of 19F NMR spectroelectrochemistry, which promises to be an important method for the elucidation of redox mechanisms of fluoroorganic compounds. Additionally, we demonstrate the importance of combining different spectroelectrochemical methods and quantitative analysis of the transferred charge and spin numbers in the determination of the redox mechanism.

 Please wait while we load your content...
Please wait while we load your content...