Glucopyranosyl-1,4-dihydropyridine as a new fluorescent chemosensor for selective detection of 2,4,6-trinitrophenol†
Abstract
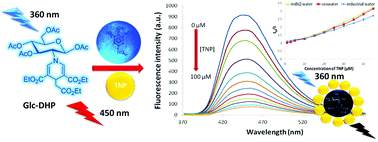
Glucopyranosyl-1,4-dihydropyridine (Glc-DHP) was synthesized as a new fluorescent chemosensor via cyclotrimerization of the β-amino acrylate in the presence of TiCl4. This DHP derivative is soluble in aqueous medium and the solution gives a blue fluorescence signal with a quantum yield of 29%. The fluorescence signal of Glc-DHP was selectively quenched by 2,4,6-trinitrophenol (TNP) with a quenching coefficient (Ksv) of 4.47 × 104 and at one of the best reported detection limits of 0.94 μM. The quenching mechanism was confirmed to be of the static type at the low concentration region (less than 50 μM) with the significant quenching effect of competitive absorption starting from the concentration of 50 μM. Even in the real sample (seawater and industrial water), the quenching efficiencies of TNP on the fluorescence emission of Glc-DHP were proven to be at the same level with that of the test in pure water, demonstrating the practicability of the detection. Furthermore, a fluorescent paper sensor could be prepared by immersing the paper into the Glc-DHP solution. The fluorescence of the paper sensor disappeared either by writing with TNP solution or by exposure to TNP vapor. This detection could be observed by the naked eye under black light. The pH effect was proven to be a substantial factor in the quenching mechanism, providing an accurate determination of TNP, 2,4-dinitrophenol (DNP) and 4-nitrophenol (4NP) in real mixed-samples.

 Please wait while we load your content...
Please wait while we load your content...