Quantitative prediction of enantioseparation using β-cyclodextrin derivatives as chiral selectors in capillary electrophoresis†
Abstract
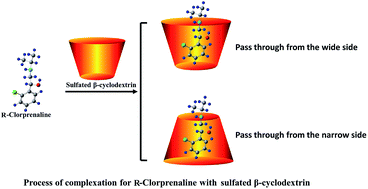
β-Cyclodextrin derivatives as chiral selectors are becoming increasingly important for enantioseparations in capillary electrophoresis (CE). Nevertheless, there are some enormous challenges in choosing effective selectors from a variety of compounds, and up to now no systematic quantitative studies for predicting the possibility of enantiomeric separation before CE experiments have been reported. In this paper, in order to resolve previous confusions, we investigated the enantioseparations of ten chiral drugs using a method of combining experiments with theoretical calculations. MMFF, PM3, DFT and ONIOM2 methods were simultaneously utilized during the course of our computer simulations. The results indicated that a specific value of greater than or approximately equal to 6 kJ mol−1 for the interaction energy difference (ΔΔE) between a pair of enantiomers with a selector is required in order to achieve enantiomeric separation. This discovery offers a meaningful reference to predict enantiomeric separations, so as to design and synthesize some more efficient chiral selectors.

 Please wait while we load your content...
Please wait while we load your content...