Design and study of the efflux function of the EGFP fused MexAB-OprM membrane transporter in Pseudomonas aeruginosa using fluorescence spectroscopy†
Abstract
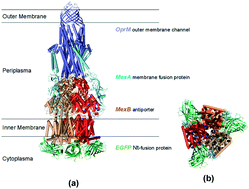
Multidrug membrane transporters (efflux pumps) can selectively extrude a variety of structurally and functionally diverse substrates (e.g., chemotoxics, antibiotics), leading to multidrug resistance (MDR) and ineffective treatment of a wide variety of diseases. In this study, we have designed and constructed a fusion gene (egfp-mexB) of N-terminal mexB with C-terminal egfp, inserted it into a plasmid vector (pMMB67EH), and successfully expressed it in the ΔMexB (MexB deletion) strain of Pseudomonas aeruginosa to create a new strain that expresses MexA-(EGFP-MexB)-OprM. We characterized the fusion gene using gel electrophoresis and DNA sequencing, and determined its expression in live cells by measuring the fluorescence of EGFP in single live cells using fluorescence microscopy. Efflux function of the new strain was studied by measuring its accumulation kinetics of ethidium bromide (EtBr, a pump substrate) using fluorescence spectroscopy, which was compared with cells (WT, ΔMexM, ΔABM, and nalB1) with various expression levels of MexAB-OprM. The new strain shows 6-fold lower accumulation rates of EtBr (15 μM) than ΔABM, 4-fold lower than ΔMexB, but only 1.1-fold higher than WT. As the EtBr concentration increases to 40 μM, the new strain has nearly the same accumulation rate of EtBr as ΔMexB, but 1.4-fold higher than WT. We observed the nearly same level of inhibitory effect of CCCP (carbonyl cyanide-m-chlorophenylhydrazone) on the efflux of EtBr by the new strain and WT. Antibiotic susceptibility study shows that the minimum inhibitory concentrations (MICs) of aztreonam (AZT) and chloramphenicol (CP) for the new strain are 6-fold or 3-fold lower than WT, respectively, and 2-fold higher than those of ΔMexB. Taken together, the results suggest that the fusion protein partially retains the efflux function of MexAB-OprM. The modeled structure of the fusion protein shows that the position and orientation of the N-terminal fused EGFP domain may either partially block the translocation pore or restrict the movement of the individual pump domains, which may lead to partially restricted efflux activity.

 Please wait while we load your content...
Please wait while we load your content...