IR action spectroscopy shows competitive oxazolone and diketopiperazine formation in peptides depends on peptide length and identity of terminal residue in the departing fragment†
Abstract
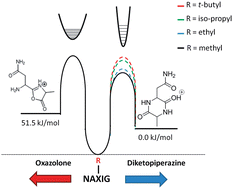
The interplay between the entropically and enthalpically favored products of peptide fragmentation is probed using a combined experimental and theoretical approach. These b2 ion products can take either an oxazolone or diketopiperazine structure. Cleavage after the second amide bond is often a favorable process because the products are small ring structures that are particularly stable. These structures are structurally characterized by action IRMPD spectroscopy and semi-quantified using gas-phase hydrogen–deuterium exchange. The formation of the oxazolone and diketopiperazine has been thought to be largely governed by the identity of the first two residues at the N-terminus of the peptide. We show here that the length of the precursor peptide and identity of the third residue play a significant role in the formation of the diketopiperazine structure in peptides containing an N-terminal asparagine residue. This is additionally the first instance showing an N-terminal residue with an amide side chain can promote formation of the diketopiperazine b2 ion structure.

 Please wait while we load your content...
Please wait while we load your content...