Characterization of new N,O-dialkyl phosphoramidate-bonded stationary phases for reversed-phase HPLC – retention and selectivity
Abstract
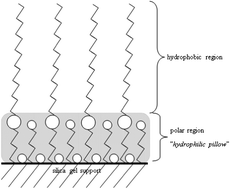
The chromatographic properties of new N,O-dialkyl phosphoramidate stationary phases were studied. Obtained stationary phases containing C10 (Amino-P-C10) and C18

 Please wait while we load your content...
Please wait while we load your content...