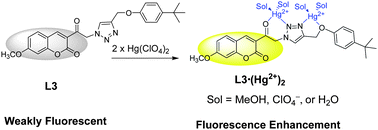
A series of triazolyl coumarin derivatives L1–L4, with and without spacer groups between the coumarin and the triazole groups, were synthesized as fluorescent sensors to study their binding ability and selectivity toward metal ions. Ligand L3, which contains an acetyl linker between the triazole and the coumarin, exhibited a high selectivity toward Hg2+ in polar protic solvents MeOH–CHCl3 (9 : 1, v/v) with fluorescent enhancement, furthermore, it was found to bind two Hg2+ at a high concentration (>12.5 mM) of Hg(ClO4)2. In contrast, L4, in which position 4 of the triazole unit was replaced by a benzyl group instead of the 4-tert-butylphenoxymethyl group used in L1–L3, showed a binding stoichiometry toward only one Hg2+. On the basis of the fluorescent sensing, IR, and 1H NMR titration results of ligands L1–L4, we proposed that not only the acetyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O but also the ether group of the 4-tert-butylphenoxymethyl of L3 assisted the triazole nitrogen atoms in the complexation of Hg2+ to form a 1 : 2 complex (L3·(Hg2+)2).
O but also the ether group of the 4-tert-butylphenoxymethyl of L3 assisted the triazole nitrogen atoms in the complexation of Hg2+ to form a 1 : 2 complex (L3·(Hg2+)2).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O but also the ether group of the 4-tert-butylphenoxymethyl of L3 assisted the
O but also the ether group of the 4-tert-butylphenoxymethyl of L3 assisted the 

 Please wait while we load your content...
Please wait while we load your content...