Intermolecular approach to metal ion indicators based on polymer phase transitions coupled to fluorescence resonance energy transfer†
Abstract
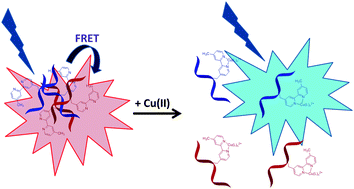
An approach to ratiometric fluorescence detection of quenching metal ions was devised by copolymerizing N-isopropylacrylamide with small percentages of bipyridine and amine monomers. The copolymer was divided into two portions. The amine group on one portion was functionalized with AlexaFluor555 (donor fluorophore) and the other with AlexaFluor647 (acceptor fluorophore). The indicator consists of a mixture of these two portions. Aggregation above the lower critical solution temperature (LCST) of this copolymer brings about a large increase in fluorescence resonance energy transfer (FRET). Addition of Cu(II) and other complexing metal ions to the aggregated copolymer introduces charge onto the backbone, causing the copolymer to redissolve with a resulting decrease in FRET. The ratio of acceptor to donor fluorescence varies with Cu(II) concentration. A plot of intensity ratio vs. pCu is sigmoidal with a log Kf of 6.1 for the Cu(II)–bipyridine complex. The data are consistent with the formation of a 1 : 1 complex. The copolymer responds to higher concentrations of other transition metal ions. The selectivity for Cu(II) is consistent with the literature values for 1 : 1 formation constants for bipyridine with metal ions.

 Please wait while we load your content...
Please wait while we load your content...