Application of different chemometric strategies to voltammetric and UV-vis spectroscopic data to obtain a complexation model: study of the Cu(ii) binding with the phytohormone 6-benzylaminopurine
Abstract
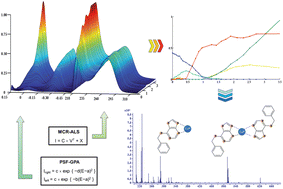
The complexation processes of Cu(II) by the phytohormone and the possible antitumoral agent 6-benzylaminopurine (BAP) were studied by Differential Pulse Polarography (DPP) and ultraviolet-visible spectroscopy (UV-vis) in combination with chemometric programs such as Multivariate Curve Resolution by Alternating Least-Squares (MCR-ALS) and Gaussian Peak Adjustment (GPA). All data confirm the formation of the predominant 1 : 1 and 1 : 2 Cu2+ : BAP complexes. The corroboration of the presented stoichiometries was performed by ElectroSpray Ionization-Mass Spectrometric (ESI-MS) experiments of the Cu(II)–BAP system at different ratios.

 Please wait while we load your content...
Please wait while we load your content...