Direct determination of free metal concentration by implementing stripping chronopotentiometry as the second stage of AGNES†
Abstract
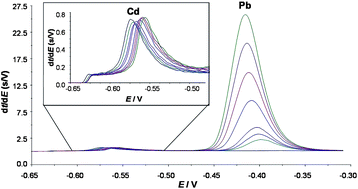
The electroanalytical technique Absence of Gradients and Nernstian Equilibrium Stripping (AGNES) has been extended by applying stripping

 Please wait while we load your content...
Please wait while we load your content...