Chronocoulometric determination of nitrate on silver electrode and sodium hydroxide electrolyte
Abstract
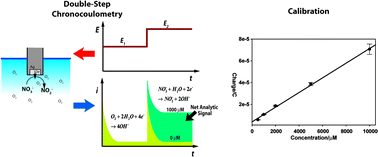
An electrochemical system that consists of a silver electrode in 0.01 M sodium hydroxide electrolyte was investigated in an effort to develop a sensitive in situ analytical method for nitrate. Cyclic voltammetry demonstrated that the proposed system has a high normalized sensitivity (2.47 A s1/2 V−1/2 M−1 cm−2), compared to more complex electroanalytical schemes. Double-potential-step chronocoulometry was used to maximize the signal-to-noise ratio (SNR), and minimize interference from dissolved oxygen in the electrolyte. The integration period for double-potential-step chronocoulometry was determined by optimizing the extended Cottrell equation. The integrated current is proportional to nitrate up to 10 mM and the average detection limit is approximately 1.7 μM. Dissolved oxygen does not degrade performance. To examine the potential interference of other ions when analyzing nitrate, we measured the electrode response to 1000 μM each of NO2−, Cl−, PO43−, SO42−, F−, CO32−, BO2−, K+, Ca2+, and Sr2+ with and without 1000 μM nitrate. Interference is negligible for most of the ions when nitrate is absent (i.e. <1% of the response to equimolar nitrate). However, interference is substantial (>20% increase or decrease in the electrode response to nitrate) for PO43−, Ca2+, and Sr2+ when equimolar nitrate is present.

 Please wait while we load your content...
Please wait while we load your content...