The product of NH3 loss from gas phase protonated tyrosine
Abstract
The lowest energy unimolecular dissociation product channel of protonated tyrosine, [Tyr + H]+, is loss of NH3. The structure of the [Tyr − NH3 + H]+ ion is still debated; past calculations suggest that the global minimum benzyl cation form is only accessible via a relatively high barrier and that a lower energy pathway to formation of the higher-energy phenonium isomer is likely to occur via collision-induced dissociation (CID). To resolve this open question, [Tyr − NH3 + H]+ was studied computationally and experimentally using ion mobility spectrometry, ultraviolet photodissociation (UVPD) spectroscopy, and infrared ion spectroscopy (IRIS). Traveling wave ion mobility spectrometry (TWIMS) yields a collision cross section of ΩN2 = 130.0 ± 1.4 Å2, which compares favorably with computed values of 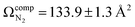 and
and 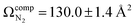 for the benzyl cation and phenonium products, respectively. Differential mobility spectrometry and mass spectrometry were used to mobility- and mass-select [Tyr + H]+ prior to producing [Tyr − NH3 + H]+via CID and subsequently measuring its UVPD spectrum. Similarly, [Tyr − NH3 + H]+ was produced via CID prior to measuring its IRIS spectrum. The UVPD and IRIS spectra indicate that the phenonium ion is the major product formed via CID.
for the benzyl cation and phenonium products, respectively. Differential mobility spectrometry and mass spectrometry were used to mobility- and mass-select [Tyr + H]+ prior to producing [Tyr − NH3 + H]+via CID and subsequently measuring its UVPD spectrum. Similarly, [Tyr − NH3 + H]+ was produced via CID prior to measuring its IRIS spectrum. The UVPD and IRIS spectra indicate that the phenonium ion is the major product formed via CID.



 Please wait while we load your content...
Please wait while we load your content...