Regulating the content of Cu+ in Cu/CeO2 catalysts for the reverse water–gas shift reaction
Abstract
The prominence of Cu/CeO2 catalysts in the RWGS reaction stems from Cu+ species and Cu–O–Ce solid solution (active toward both CO2 and H2). However, a reduction process is required for catalyst activation, which potentially causes excessive Cu+ reduction to Cu0 and damage to the Cu–O–Ce structure. Herein, the Cu+ content in Cu/CeO2 is tuned by adjusting the H2/Ar ratio during the reduction process, while ensuring the preservation of the Cu–O–Ce solid solution structure. The catalyst reduced in an atmosphere with a H2 molar fraction of 0.25 exhibits the maximum content of oxygen vacancies and retains the highest surface Cu+ proportion (76.93%, as determined by Auger spectroscopy). This catalyst exhibits high CO2 conversion (49.89% at 500 °C, H2/CO2 = 4 : 1) and low activation energy in the RWGS reaction. The Cu+ species and oxygen vacancies are essential to activating CO2 and H2, and the RWGS reaction follows the formate pathway with 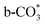 and HCOO* acting as intermediates. This study offers guidance for designing Cu-based catalysts for the RWGS reaction by exploring pretreatment conditions.
and HCOO* acting as intermediates. This study offers guidance for designing Cu-based catalysts for the RWGS reaction by exploring pretreatment conditions.



 Please wait while we load your content...
Please wait while we load your content...