Pressure-driven phase transformations on Mg3Ca(CO3)4 huntite carbonate†
Abstract
Magnesium and calcium carbonate minerals are significant reservoirs of Earth's carbon and understanding their behavior under different conditions is crucial for elucidating the mechanisms of deep carbon storage. Huntite, Mg3Ca(CO3)4, is one of the two stable calcium magnesium carbonate phases, together with dolomite. The distinctive cation coordination environment of Ca atoms compared to calcite-type and dolomite structures makes huntite a comparatively less dense phase. Here we examine the behavior of a polycrystalline natural huntite sample under room-temperature compression up to 38 GPa. Synchrotron X-ray diffraction and Raman spectroscopy experiments were carried out in a diamond-anvil cell using He as a highly hydrostatic pressure transmitting medium. XRD results suggest that the initial R32 huntite structure persists up to 21 GPa. The Raman experiment agrees with this result but also suggests the appearance of structural defects from 10 GPa on. Birch–Murnaghan equation of state parameters were fit to the pressure–volume huntite data resulting in zero-pressure volume V0 of 611.7(2) Å3, a bulk modulus B0 of 99.5(11) GPa and a pressure derivative of the bulk modulus of 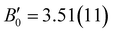 . At 21 GPa, huntite transforms to another trigonal phase (R3), designated here as huntite II. This phase persists up to at least 38 GPa, the maximum pressure reached in this study. The major structural differences between huntite and the huntite-II phase involve the tilting of the [CO3] units with respect to the basal plane and a rotation, which cause a progressive change in the coordination number of the Ca atoms, from 6 to 9. DFT calculations complement the experimental data, providing new insights into the structural response to high-pressure conditions of this magnesium–calcium double carbonate mineral.
. At 21 GPa, huntite transforms to another trigonal phase (R3), designated here as huntite II. This phase persists up to at least 38 GPa, the maximum pressure reached in this study. The major structural differences between huntite and the huntite-II phase involve the tilting of the [CO3] units with respect to the basal plane and a rotation, which cause a progressive change in the coordination number of the Ca atoms, from 6 to 9. DFT calculations complement the experimental data, providing new insights into the structural response to high-pressure conditions of this magnesium–calcium double carbonate mineral.



 Please wait while we load your content...
Please wait while we load your content...