Strain-controlled spin regulation in Fe–N–C catalysts for enhanced oxygen reduction reaction activity†
Abstract
Fe-embedded N-doped graphene (Fe–N–C) stands out as a promising electrocatalyst for the oxygen reduction reaction (ORR), a critical process in fuel cell technologies. The role of spin multiplicity at the Fe center and its influence on ORR intermediates, particularly the spin crossover effect, is a pivotal yet underexplored aspect of spin-related electrocatalysis. Unraveling the nuances of spin multiplicity and its manipulation holds the key to the next generation of Fe-based spin electrocatalysts for renewable energy. Our study employs a first-principles density functional theory (DFT) approach to dissect the electronic configurations across various spin states of ORR intermediates. Our wavefunction and bond analysis unveil a critical mechanism: the spin electron transfer from the nonbonding dx2–y2 orbital to the antibonding 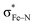 orbital, which dominantly dictates the spin state transition in ORR intermediates on the Fe–N–C catalyst. This insight is further complemented by the discovery that the electronic occupation of d–p π bonds, formed by X–Fe–Y (X/Y: *OH, *O, and *OOH), is insensitive to the IS–HS transition of adsorbates. Another novel finding of this research is the strain-induced modulation of spin states within Fe–N–C. We demonstrate that increasing strain drives a transition from an intermediate spin (IS) to a high spin (HS) state in FeN4 and its ORR intermediates, effectively suppressing ligand-induced spin crossover. Notably, a unique behavior is observed in five-coordinate FeN4OH and FeN4OOH, where strain prompts a return to IS due to electron injection into the dxy orbital. Our simulation of polarization current density reveals a direct correlation between applied strain and ORR onset potential. Specifically, at +8% strain, the onset potential is remarkably enhanced to 0.98 V vs. SHE due to the dominant active moiety of FeNHS4*OH, underscoring the role of spin state modulation in weakening the Fe–O bond and reducing the desorption energy of *OH. This work not only elucidates the origin of spin multiplicity in ORR intermediates on Fe–N–C but also establishes strain-controlled spin regulation as a viable strategy for tuning ORR activity. Our findings offer a significant insight into the design of spin-related electrocatalysts, paving the way for more efficient and sustainable energy technologies.
orbital, which dominantly dictates the spin state transition in ORR intermediates on the Fe–N–C catalyst. This insight is further complemented by the discovery that the electronic occupation of d–p π bonds, formed by X–Fe–Y (X/Y: *OH, *O, and *OOH), is insensitive to the IS–HS transition of adsorbates. Another novel finding of this research is the strain-induced modulation of spin states within Fe–N–C. We demonstrate that increasing strain drives a transition from an intermediate spin (IS) to a high spin (HS) state in FeN4 and its ORR intermediates, effectively suppressing ligand-induced spin crossover. Notably, a unique behavior is observed in five-coordinate FeN4OH and FeN4OOH, where strain prompts a return to IS due to electron injection into the dxy orbital. Our simulation of polarization current density reveals a direct correlation between applied strain and ORR onset potential. Specifically, at +8% strain, the onset potential is remarkably enhanced to 0.98 V vs. SHE due to the dominant active moiety of FeNHS4*OH, underscoring the role of spin state modulation in weakening the Fe–O bond and reducing the desorption energy of *OH. This work not only elucidates the origin of spin multiplicity in ORR intermediates on Fe–N–C but also establishes strain-controlled spin regulation as a viable strategy for tuning ORR activity. Our findings offer a significant insight into the design of spin-related electrocatalysts, paving the way for more efficient and sustainable energy technologies.



 Please wait while we load your content...
Please wait while we load your content...