Locating manganese vanadate phase with PO43−-modified Mn2+–O–V5+ motifs optimized for catalytic NOX and poison abatement under oxidative wet conditions†
Abstract
Metal oxide crystallites possess characteristic structural motifs, which can act as active centers to direct the activities, selectivities, and sustainabilities of target reactions. In this context, Mn2+–O–V5+ motifs are essential to construct Mn2+/V5+-centered sub-units for manganese vanadate phases (MnXV2OX+5; X = 1–3). Surface Mn2+–O–V5+ motifs are readily fragmented to bear labile oxygens (OL) and Lewis acidic (LA) defects adjacent to oxygen vacancies (OV) functioning as reservoirs of mobile oxygens (OM). The LA defects possess high affinity for O2/H3PO4 poison included in NOX/SO2-containing, wet feed gases. Meanwhile, the LA defects afford PO43− functionalities, whose terminal P5+–O2− bonds act as Brønsted acidic species (BA−–H+) upon protonation. The resulting BA−–H+-rich, fragmented Mn2+–O–V5+ motifs are particularly conducive to activate the selective reduction of NOX (SCR) via the Eley–Rideal (ER) model or the pyrolysis of ammonium (bi-)sulfate (AS/ABS) poisons via the pyrosulfate disintegration model. Marked acceleration of the ER and pyrosulfate fragmentation models hinge on the energy barrier (EBARRIER) reduction/collision frequency 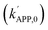 elevation/strong hydrophobicity, whose trends versus X anticipated using the local Mn2+–O–V5+ connectivities in the Mn2+-centered sub-units are opposite to those in their V5+-centered counterparts. Nonetheless, the Mn2+/V5+-centered sub-units were verified to impart Mn2+–O–V5+ motifs that were highly associated and similarly contributed to disclosing the trends of the kinetic parameters
elevation/strong hydrophobicity, whose trends versus X anticipated using the local Mn2+–O–V5+ connectivities in the Mn2+-centered sub-units are opposite to those in their V5+-centered counterparts. Nonetheless, the Mn2+/V5+-centered sub-units were verified to impart Mn2+–O–V5+ motifs that were highly associated and similarly contributed to disclosing the trends of the kinetic parameters 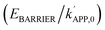 , hydrophobicity, or amounts/strengths of the BA−–H+/redox sites (OL/OV/OM) versus X, throughout which X = 1–2 were more desired than X = 3 except for the
, hydrophobicity, or amounts/strengths of the BA−–H+/redox sites (OL/OV/OM) versus X, throughout which X = 1–2 were more desired than X = 3 except for the  values alongside with a higher hydrophobic surface area provided by X = 1 compared to those provided by X = 2–3. Notably, Sb2O5-promoted Mn1V2O6 subjected to PO43− modification (Mn1–Sb–P) was superior to WO3-promoted V2O5 (commercial control) or SOA2−-modified analogue (Mn1–Sb–S) in achieving higher activities and/or maximum-obtainable performance for the SCR or AS/ABS pyrolysis, as substantiated by controlled or 18O2-labelled runs under kinetic/diffusion-limited domains.
values alongside with a higher hydrophobic surface area provided by X = 1 compared to those provided by X = 2–3. Notably, Sb2O5-promoted Mn1V2O6 subjected to PO43− modification (Mn1–Sb–P) was superior to WO3-promoted V2O5 (commercial control) or SOA2−-modified analogue (Mn1–Sb–S) in achieving higher activities and/or maximum-obtainable performance for the SCR or AS/ABS pyrolysis, as substantiated by controlled or 18O2-labelled runs under kinetic/diffusion-limited domains.



 Please wait while we load your content...
Please wait while we load your content...