Dehydration kinetics of the synthesis of high-nickel cathode materials used in lithium ion batteries†
Abstract
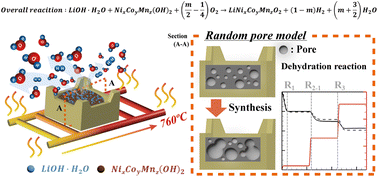
Dehydration reactions of cathode precursors such as lithium hydroxide monohydrate (LiOH·H2O) and transition metal hydroxide (NixCoyMnz(OH)2) cause considerable difficulties in temperature control during the synthesis of cathode materials used in lithium ion batteries. The failure in temperature control results in the incomplete removal of moisture from a product. Thus, the kinetics of dehydration reactions should be considered when controlling the temperature and moisture in the Roller Hearth Kiln. In this study, the dehydration reactions of each precursor during the Ni-rich cathode material synthesis are studied using in situ X-ray diffraction and a thermogravimetric analyzer connected with a mass spectrometer. Two dehydration reactions for LiOH·H2O are identified in the Ar atmosphere. A two-step decomposition mechanism for NixCoyMnz(OH)2 is proposed, with one reaction producing moisture, hydrogen and oxygen and the other producing oxygen. A random pore model (RPM) is used to examine the three moisture-producing reactions and to obtain their kinetic parameters. The intrinsic kinetic parameters of the three dehydration reactions are calculated by performing isothermal thermogravimetric analysis and using the structural parameters for each precursor. The RPM and experimental results show good agreement for each precursor and the mixture in terms of the mass ratio of LiOH·H2O to NixCoyMnz(OH)2 (L/N ratio).


 Please wait while we load your content...
Please wait while we load your content...