Combining total synthesis and genetic engineering to probe dihydropyran formation in ambruticin biosynthesis†
Abstract
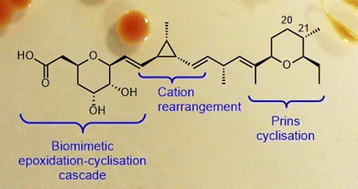
The ambruticins are a family of potent antifungal polyketide derived natural products isolated from the myxobacterium Sorangium cellulosum. Their unusual structures include a trisubstituted cyclopropyl group and two oxygen heterocycles, a tetrahydropyran (THP) and dihydropyran (DHP). Herein we report a flexible modular approach for the total synthesis of ambruticins which is used to prepare ambruticins F and S as well as in the first total synthesis of 20,21-dihydroambruticin F. The flexible strategy unites 3 fragments via Julia–Kocienski olefinations and provides important standards for investigation of dihydropyran formation in ambruticin biosynthesis. Cultures of wild-type S. cellulosum So ce10 produce mainly ambruticin S and the VS series of metabolites. An efficient electroporation method enabled gene knockout experiments which revealed that the ΔambP-S mutant of S. cellulosum accumulated the bisTHP polyketide 20,21-dihydroambruticin F. In contrast, the ΔambN-S mutant gave ambruticin F with the 20,21-alkene as the major metabolite confirming that AmbP and AmbO (a Rieske enzyme and flavin-dependent monooxygenase respectively) are implicated in 20,21-alkene formation. The results of feeding studies to a Sorangium strain containing only ambP and ambO are in accord with formation of the 20,21-alkene occurring prior to generation of the C3 to C7 dihydroxylated tetrahydropyran in ambruticin biosynthesis.


 Please wait while we load your content...
Please wait while we load your content...