Access to chiral dihydrophenanthridines via a palladium(0)-catalyzed Suzuki coupling and C–H arylation cascade reaction using new chiral-bridged biphenyl bifunctional ligands†
Abstract
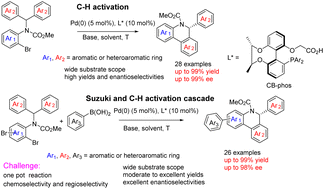
A class of chiral-bridged biphenyl phosphine-carboxylate bifunctional ligands CB-Phos has been developed and successfully applied to Pd(0)-catalyzed single enantioselective C–H arylation and a one pot cascade reaction involving Suzuki cross-coupling and C–H arylation. The catalytic system provides a new and convenient way for the synthesis of versatile chiral dihydrophenanthridines with rich structures and broad functional group tolerance. Good to excellent yields with high enantioselectivities were generally achieved. The reaction mechanism of the cascade reaction was also preliminarily discussed.


 Please wait while we load your content...
Please wait while we load your content...