Copper-catalyzed carbonylative multi-component borylamidation of alkenes for synthesizing γ-boryl amides with CO as both methylene and carbonyl sources†
Abstract
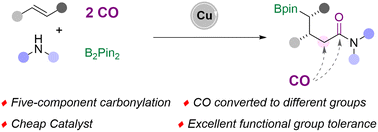
A multi-component carbonylation reaction is an efficient strategy for the synthesis of valuable carbonyl compounds from simple and readily available substrates. However, there remain challenges in carbonylation reactions where two CO molecules are converted to different groups in the target product. Considering the merit of complex amides, we reported here a copper-catalyzed multi-component borylamidation for the synthesis of γ-boryl amides. This method provides access to a wide range of functional γ-boryl amides from alkenes, amines, B2pin2, and CO with good yields and excellent diastereomeric ratios. Notably, two CO molecules were converted to methylene and carbonyl groups in the target amides. A series of amines were successfully involved in the transformation, including arylamines, aliphatic amines, and hydrochloride salts of secondary aliphatic amines.


 Please wait while we load your content...
Please wait while we load your content...