Evidence for Suzuki–Miyaura cross-couplings catalyzed by ligated Pd3-clusters: from cradle to grave†
Abstract
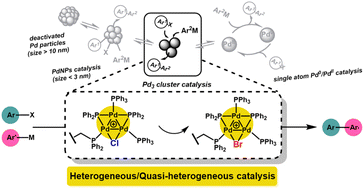
Pdn clusters offer unique selectivity and exploitable reactivity in catalysis. Understanding the behavior of Pdn clusters is thus critical for catalysis, applied synthetic organic chemistry and greener outcomes for precious Pd. The Pd3 cluster, [Pd3(μ-Cl)(μ-PPh2)2(PPh3)3][Cl] (denoted as Pd3Cl2), which exhibits distinctive reactivity, was synthesized and immobilized on a phosphine-functionalized polystyrene resin (denoted as immob-Pd3Cl2). The resultant material served as a tool to study closely the role of Pd3 clusters in a prototypical Suzuki–Miyaura cross-coupling of 4-fluoro-1-bromobenzene and 4-methoxyphenyl boronic acid at varying low Pd ppm concentrations (24, 45, and 68 ppm). Advanced heterogeneity tests such as Hg poisoning and the three-phase test showed that leached mononuclear or nanoparticulate Pd are unlikely to be the major active catalyst species under the reaction conditions tested. EXAFS/XANES analysis from (pre)catalyst and filtered catalysts during and after catalysis has shown the intactness of the triangular structure of the Pd3X2 cluster, with exchange of chloride (X) by bromide during catalytic turnover of bromoarene substrate. This finding is further corroborated by treatment of immob-Pd3Cl2 after catalyzing the Suzuki–Miyaura reaction with excess PPh3, which releases the cluster from the polymer support and so permits direct observation of [Pd3(μ-Br)(μ-PPh2)2(PPh3)3]+ ions by ESI-MS. No evidence is seen for a proposed intermediate in which the bridging halogen on the Pd3 motif is replaced by an aryl group from the organoboronic acid, i.e. formed by a transmetallation-first process. Our findings taken together indicate that the ‘Pd3X2’ motif is an active catalyst species, which is stabilized by being immobilized, providing a more robust Pd3 cluster catalyst system. Non-immobilized Pd3Cl2 is less stable, as is followed by stepwise XAFS of the non-immobilized Pd3Cl2, which gradually changes to a species consistent with ‘Pdx(PPh3)y’ type material. Our findings have far-reaching future implications for Pd3 cluster involvement in catalysis, showing that immobilization of Pd3 cluster species offers advantages for rigorous mechanistic examination and applied chemistries.
- This article is part of the themed collection: 2024 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...