Alkyl sulfonate surfactant mediates electroreduction of carbon dioxide to ethylene or ethanol over hydroxide-derived copper catalysts†
Abstract
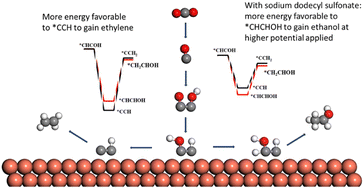
For CO2 electroreduction (CO2ER) to C2 compounds, it is generally accepted that the formation of ethylene and ethanol shares the same intermediate, *HCCOH. The majority of studies have achieved high faradaic efficiency (FE) towards ethylene, but faced challenges to get high ethanol FE. Herein, we present an alkyl sulfonate surfactant (e.g., sodium dodecyl sulfonate, SDS) mediated CO2ER to a C2 product over an in situ generated Cu catalyst (Cu@SDS) from SDS-modified Cu(OH)2. It achieves the CO2ER to ethylene as the sole C2 product at low applied voltages with a FE of 55% at −0.6 V vs. RHE and to ethanol as the main product at potentials ≥0.7 V with a maximum FE of 64% and a total C2 FE of 86% at −0.8 V, with a current density of 231 mA cm−2 in a flow cell. Mechanism investigation indicates that SDS modifies the oxidation state of the in situ formed Cu species in Cu@SDS, thus tuning the catalyst activity for CO2ER and lowering the C–C coupling energy barrier; meanwhile, it tunes the adsorption mode of the *HCCOH intermediates on the catalyst, thus mediating the selectivity of CO2ER towards C2 products.


 Please wait while we load your content...
Please wait while we load your content...