Oxidative cleavage of ketoximes to ketones using photoexcited nitroarenes†
Abstract
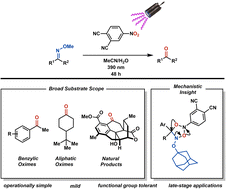
The methoxime group has emerged as a versatile directing group for a variety of C–H functionalizations. Despite its importance as a powerful functional handle, conversion of methoximes to the parent ketone, which is often desired, usually requires harsh and functional group intolerant reaction conditions. Therefore, the application of methoximes and their subsequent conversion to the corresponding ketone in a late-stage context can be problematic. Here, we present an alternative set of conditions to achieve mild and functional group tolerant conversion of methoximes to the parent ketones using photoexcited nitroarenes. The utility of this methodology is showcased in its application in the total synthesis of cephanolide D. Furthermore, mechanistic insight into this transformation obtained using isotope labeling studies as well as the analysis of reaction byproducts is provided.


 Please wait while we load your content...
Please wait while we load your content...