Electrosynthesis of iminophosphoranes and applications in nickel catalysis†
Abstract
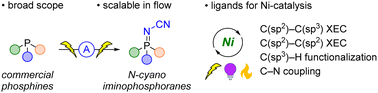
P(V) iminophosphorane compounds are accessed via electrochemical oxidation of commercially available P(III) phosphines, including mono-, di- and tri-dentate phosphines, as well as chiral phosphines. The reaction uses inexpensive bis(trimethylsilyl)carbodiimide as an efficient and safe aminating reagent. DFT calculations, cyclic voltammetry, and NMR studies provide insight into the reaction mechanism. The proposed mechanism reveals a special case of sequential paired electrolysis. DFT calculations of the frontier orbitals of an iminophosphorane are compared with those of the analogous phosphines and phosphine oxides. X-ray crystallographic studies of the ligands as well as a Ni-coordination complex provide structural insight for these ligands. The utility of these iminophosphoranes as ligands is demonstrated in nickel-catalyzed cross-electrophile couplings including C(sp2)–C(sp3) and C(sp2)–C(sp2) couplings, an electrochemically driven C–N cross-coupling, and a photochemical arylative C(sp3)–H functionalization. In some cases, these new ligands provide improved performance over commonly used sp2-N-based ligands (e.g. 4,4′-di-tert-butyl-2,2′-bipyridine).


 Please wait while we load your content...
Please wait while we load your content...