Manganese-catalyzed base-free addition of saturated nitriles to unsaturated nitriles by template catalysis†
Abstract
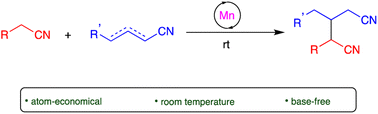
The coupling of mononitriles into dinitriles is a desirable strategy, given the prevalence of nitrile compounds and the synthetic and industrial utility of dinitriles. Herein, we present an atom-economical approach for the heteroaddition of saturated nitriles to α,β- and β,γ-unsaturated mononitriles to generate glutaronitrile derivatives using a catalyst based on earth-abundant manganese. A broad range of such saturated and unsaturated nitriles were found to undergo facile heteroaddition with excellent functional group tolerance, in a reaction that proceeds under mild and base-free conditions using low catalyst loading. Mechanistic studies showed that this unique transformation takes place through a template-type pathway involving an enamido complex intermediate, which is generated by addition of a saturated nitrile to the catalyst, and acts as a nucleophile for Michael addition to unsaturated nitriles. This work represents a new application of template catalysis for C–C bond formation.


 Please wait while we load your content...
Please wait while we load your content...