Strain induced reactivity of cyclic iminoboranes: the (2 + 2) cycloaddition of a 1H-1,3,2-diazaborepine with ethene†
Abstract
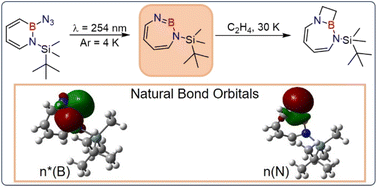
Iminoboranes have gathered immense attention due to their reactivity and potential applications as isoelectronic and isosteric alkynes. While cyclic alkynes are well investigated and useful reagents, cyclic iminoboranes are underexplored and their existence was inferred only via trapping experiments. We report the first direct spectroscopic evidence of a cyclic seven-membered iminoborane, 1-(tert-butyldimethylsilyl)-1H-1,3,2-diazaborepine 2, under cryogenic matrix isolation conditions. The amino-iminoborane 2 was photochemically generated in solid argon at 4 K from 2-azido-1-(tert-butyldimethylsilyl)-1,2-dihydro-1,2-azaborinine (3) and was characterized using FT-IR, UV-vis spectroscopy, and computational chemistry. The characteristic BN stretching vibration (1751 cm−1) is shifted by about 240 cm−1 compared to linear amino-iminoboranes indicating a significant weakening of the bond. The Lewis acidity value determined computationally (LAB = 9.1 ± 2.6) is similar to that of boron trichloride, and twelve orders of magnitude lower than that of 1,2-azaborinine (BN-aryne, LAB = 21.5 ± 2.6), a six-membered cyclic iminoborane. In contrast to the latter, the reduced ring strain of 2 precludes nitrogen fixation, but it unexpectedly allows facile (2 + 2) cycloaddition reaction with C2H4 under matrix isolation conditions at 30 K.


 Please wait while we load your content...
Please wait while we load your content...