A study on the reaction pathway and mechanism of urea alcoholysis by the disassociation and conjugation of groups†
Abstract
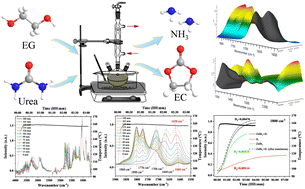
The chemical conversion of alcohols to carbonates, which are high value-added products, is considered a green transformation route; however, the underlying reaction mechanism remains unclear and needs further study. Considering the synthesis of ethylene carbonate (EC) from urea and ethylene glycol (EG) as a model reaction using tetrabutylphosphonium bromide ([P4444][Br]) and zinc bromide (ZnBr2) as the binary catalyst, the yield and selectivity of EC could reach 83.07% and 95.38%, respectively. Subsequently, the qualitative and quantitative analyses of major components in the reaction were performed via GC-MS, 1H NMR, 13C NMR, and kinetics studies. Additionally, three catalysis processes catalyzed by [P4444][Br], ZnBr2, and [P4444][Br]/ZnBr2 catalysts were detected by in situ FT-IR spectra. Meanwhile, the possible reaction pathway and mechanism of the formation of EC from urea and EG was systematically studied according to the variation tendencies of a range of functional groups. The introduction of a catalyst as a judging criterion for determining the rates of group disassociation and conjugation on substrates and products provided a new reference basis for the evaluation of catalytic reaction processes and the performance of catalysts.


 Please wait while we load your content...
Please wait while we load your content...