Aqueous solution and solid-state behaviour of l-homophenylalanine: experiment, modelling, and DFT calculations
Abstract
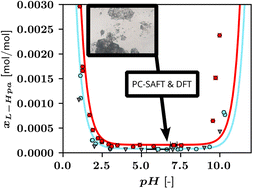
In the present study, the solid-state and aqueous solubility behaviour of L-homophenylalanine (L-Hpa) is explored. Different characterization techniques such as TG, DSC, temperature-resolved PXRD, and hot-stage microscopy were used to investigate basic thermal solid-state characteristics. Solubilities of L-Hpa in water were determined as a function of temperature and pH. Moreover, a thermodynamic model based on perturbation theory (PC-SAFT) is applied to represent the data. In addition, aqueous density data of L-Hpa were measured in a broader temperature range. To model the solubility data as a function of pH, pKa values are needed, which were accessed by employing density functional theory (DFT) calculations. The solid-state investigation did not show a simple melting process of L-Hpa, but a complete decomposition of the prevalent initial solid phase at elevated temperatures approximately above 520 K. This system exhibited extraordinarily low solubilities for an amino acid at all investigated temperatures. While the solubility does not differ from its isoelectric-point value over a wide pH range, it dramatically increases as the pH falls below 2.5 and rises above 9.5. The PC-SAFT model was able to calculate the solubilities as a function of pH and predict the density values.


 Please wait while we load your content...
Please wait while we load your content...